Beginning around 1965 researchers began to notice that people had different levels of an enzyme in their intestinal tract called intestinal alkaline phosphatase (IAP) and that the levels of this enzyme varied according to ABO blood group and secretor status.
IAP has several important functions. During fetal development, IAP is the enzyme with the highest blood concentration during the critical period when the gut lining is developing. IAP also helps to split cholesterol and long chain fatty acids from food into smaller fatty acids.
IAP helps maintain the intestinal mucosal barrier by detoxifying lipopolysaccharides (LPS), a component of Gram-negative bacterial cell walls. LPS is a potent endotoxin that can trigger inflammation if it crosses the gut barrier into the bloodstream. This protective role is vital for preventing "leaky gut" syndromes and associated conditions like inflammatory bowel disease (IBD). Studies in mice show that IAP deficiency leads to dysbiosis—an imbalance in gut flora—linked to metabolic disorders and increased susceptibility to infections like Clostridium difficile.
IAP contributes to maintaining an optimal pH in the intestinal tract by buffering the environment. This helps create conditions favorable for beneficial bacteria and digestion while discouraging pathogens that thrive in altered pH settings.
Research has linked higher IAP activity with improved outcomes in sepsis and critical illness, highlighting its broader protective role
Finally, it also enhances the absorption of calcium from food.
Type A non-secretors have the lowest levels, and type O secretors the highest, with type B's somewhere in the middle. The activity of intestinal alkaline phosphatase and serum alkaline phosphatase is strongly correlated with ABH secretor phenotypes. Independent of ABO blood group, ABH non-secretors have lower alkaline phosphatase activity than ABH secretors. It has been estimated that the serum alkaline phosphatase activity of non-secretors is only about 20% of the activity in the secretor groups. It appears likely that the ABO and secretor genes influence the rate at which the intestinal phosphatase enters the blood, or its catabolism, rather than its synthesis in the intestine.
The concentration of the intestinal phosphatase is lowest in the serum during fasting and rises after ingestion of fat, reaching a peak at about seven to eight hours. The concentration of intestinal alkaline phosphatase in human thoracic-duct lymph rises after a fatty meal; and presumably, most of the intestinal phosphatase enters the blood by way of the lymphatic system.
So, if type O secretors had higher levels of this enzyme, can assume they do a somewhat better job of digesting fats? Most fascinating, studies have shown that IAP likes a protein meal, as protein tends to switch IAP into overdrive; which may be counterintuitive, as current nutritional wisdom holds that protein intake increases bone loss due to the use of bone calcium as a pH buffer.
Paradoxically, for blood group A individuals not only are their levels of IAP lower, but evidence also suggests that the physical presence of the A antigen actually helps to inactivate what little IAP they do make.
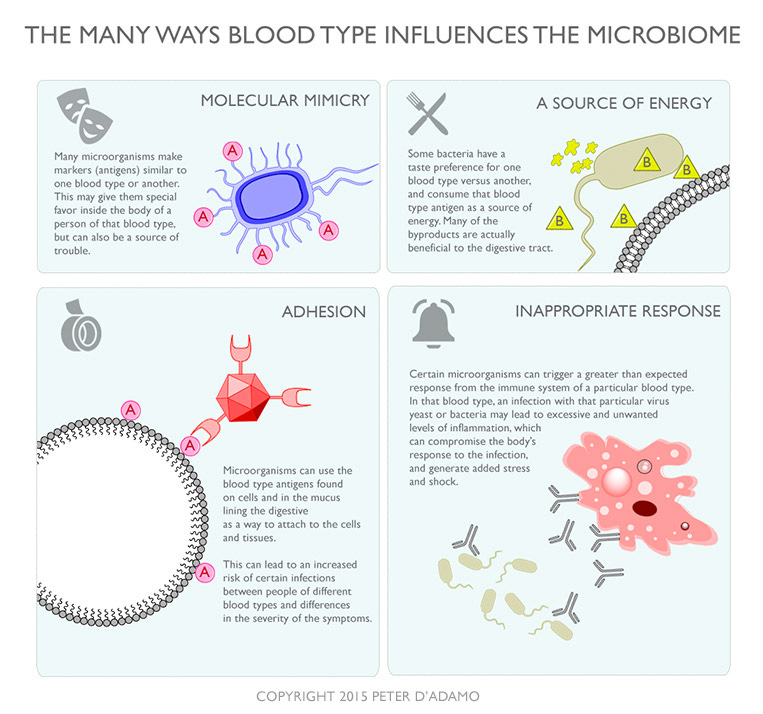
ABO blood group has been increasingly recognized as influencing the gut microbiome. These effects stem from the interaction between blood group antigens (or their absence) and microbial communities, particularly in the gastrointestinal tract, where these antigens are also expressed on mucosal surfaces and in secretions.
Blood Group A: Studies have shown enrichment of certain bacteria, such as Bacteroides species, in individuals with type A blood. This may be due to the N-acetylgalactosamine residue in the A antigen, which some microbes preferentially metabolize or bind to.
Blood Group B: Individuals with type B blood, characterized by a galactose residue, may favor bacteria like certain Lactobacillus or Prevotella strains, though data is less consistent.
Blood Group O: Lacking A or B antigens (but expressing the H antigen), type O individuals often show higher abundance of Fusobacterium and some Proteobacteria. The H antigen (fucose-based) may attract fucose-utilizing bacteria like Bacteroides thetaiotaomicron.
Blood Group AB: As a hybrid, AB individuals exhibit a mix of microbial patterns, though research is limited.